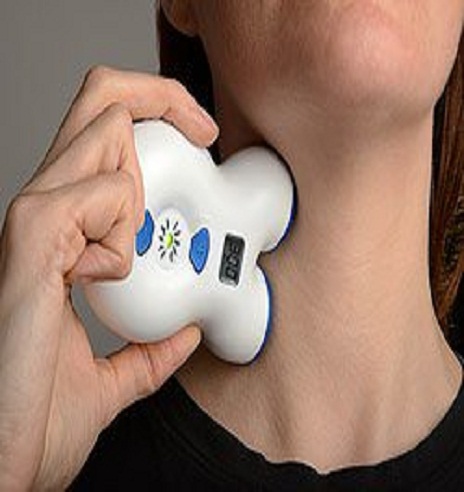
The US Food and Drug Administration (FDA) has cleared the hand-held, noninvasive vagus nerve stimulator (nVS) gammaCore (electroCore LLC) for the treatment of migraine pain in adults, the manufacturer announced earlier today.
The new 510(k) clearance will expand the device’s label from just treating episodic cluster headache pain, an indication that the FDA approved in April 2017.
After the portable device is placed over the vagus nerve in the neck, it releases a mild electrical stimulation to the nerve’s afferent fibers.
“With the FDA’s decision to release gammaCore for migraine, patients now have access to an effective and safe therapy which can be self-administered to acutely treat the pain associated with migraine,” Stephen D. Silberstein, MD, professor of neurology and director of the Headache Center at Thomas Jefferson University, Philadelphia, Pennsylvania, said in a news release.
Findings from the randomized, multicenter Prospective Study of nVNS for the Acute Treatment of Migraine (PRESTO) played a major role in the FDA’s expanded clearance, notes the manufacturer.
PRESTO Results
PRESTO included 243 patients with episodic migraine. Significantly more members of the group receiving nVNS were pain free at 30 minutes (12.7%) than those receiving a sham treatment (4.2%; P = .01). There was also a greater percentage of the nVNS group who were pain free at 60 minutes (21% vs 10%, respectively; P = .02).
Although between-group differences for being pain free at 120 minutes missed statistical significance (30.4% vs 19.7%, respectively; P = .07), significance for this outcome was found after a post hoc repeated-measures test (odds ratio, 2.3; P = .01).
The secondary endpoint of mild or no pain at 2 hours was also significantly greater in those receiving neuromodulation (40.8% vs 27.6%; P = .03), as was pain reduction at 2 hours (34.8% vs 5.4%; P = .004).
“The PRESTO data suggests that gammaCore was rapidly effective, well-tolerated, and practical for the acute treatment of episodic migraine,” principal investigator, Cristina Tassorelli, MD, director of the Headache Science Center at the C. Mondino National Neurological Institute, Pavia, Italy, in a press announcement released in 2017.
“Migraine is the third most common disease in the world, and one of the 10 most disabling diseases, which highlights a need for novel treatment options,” Dr Tassorelli added.
The device will be available commercially for the treatment of migraine headache pain in adults in the second quarter of 2018, according to the manufacturer.
It adds, though, that the safety and effectiveness of the prescription-only device have not been established in the acute treatment of chronic cluster headache or in the prophylactic treatment of chronic or episodic cluster or migraine headache.
This type of therapy is also not indicated for children; pregnant women; patients with active implantable medical devices; those with conditions such as carotid atherosclerosis, hypertension, or bradycardia/tachycardia; or those with a metallic device, such as a stent, bone plate, or bone screw near the neck. It also shouldn’t be used at the same time as the use of a mobile phone or other portable electronic device.